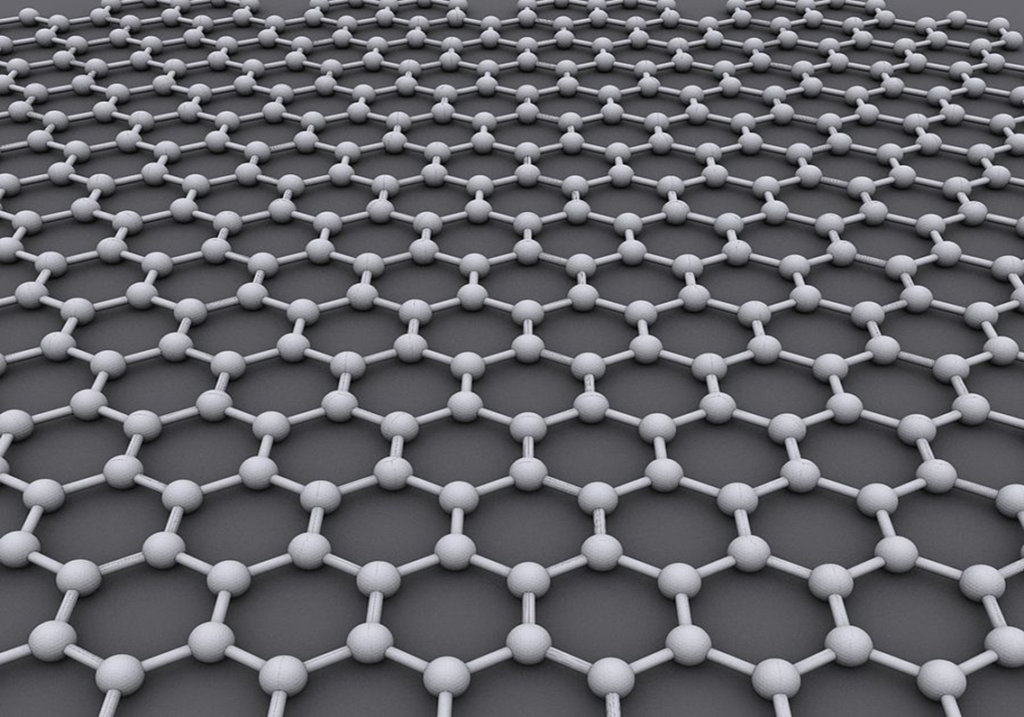
I learned this today. Graphene is a one-atom-thick layer of carbon atoms arranged in a hexagonal lattice. It is incredibly thin and incredibly strong.
In 1859, Benjamin Brodie noticed that graphite oxide contained lots of layers. In 1947, P. R. Wallace published a paper on the band structure of graphite and theorized that a single layer could be possible. Hanns-Peter Boehm came up with the name graphene in 1961. He managed to make flakes of graphite that were 3 atoms thick. In 2004, graphene was created by Andre Geim and Konstantin Novoselov. They used an exfoliation technique. That means they peeled away layers of graphite until they were left with a one-atom thick layer. They used common Scotch to do it. It took a while because a 1mm thick piece of graphite has three million layers, but it was a relatively simple process. In 2010, they won the Nobel Prize for this discovery.
So, what is so special about graphene? Because it is only one atom thick, it is incredibly light, but it has an unbelievable strength. It is the thinnest compound known to man and 1m2 weighs 0.77mg. 1g would give you 2,630m2. You need 3g to cover a soccer field. Combined with that, it is about 300 times stronger than steel. It is an excellent heat conductor, 2 times better than diamond. It conducts electricity very well, 13 times better than copper. It is almost completely transparent: absorbing only 2.3% of light. It is impermeable to gases because it is so dense. It is extremely elastic.
Graphene has a myriad of potential uses. It can be added to other materials, such as plastics and metals, to make them stronger. The potential for lighter planes, and lighter spaceflight are incredible.
Because it is so heat-conductive, it would make a great heat-spreading solution. This would be a huge advance for mobile devices or microelectronics.
Because it is thin with a huge surface-area to volume ratio it could be used in batteries or supercapacitors to increase energy storage and speed up charging. Graphene batteries are lighter and cheaper than lithium batteries, they charge faster, and they last ten times as long without losing their ability to recharge in the way that lithium does. Graphene batteries will make the electric car possible.
Graphene can be mixed with paint and painted onto any structure. It eliminates corrosion and could also be used as armor. It could replace Kevlar in bulletproof jackets or on armored vehicles.
Graphene is such an amazing material that there is pretty much no industry it won’t benefit. Being stronger and lighter, conducting more heat or electricity, and being more flexible than any other material all at the same time make it incredibly useful. Plus, carbon is the fourth most abundant element in the universe, so it is literally everywhere. It could be an eco-friendly and sustainable way of making so many things.
However, this is all well and good, but graphene needs to be mass-produced to be of any use and the Scotch tape method used in the original experiment doesn’t scale up. Graphene needs to be perfect if it is to have all of the attributes I have listed.
There are two ways of producing graphene. There is the bottom-up process, where graphene is built atom by atom. Or there is the top-down process where graphite is split into layers of graphene. The bottom-up process makes the best quality graphene, but it is very slow and expensive. The top-down process can make more graphene, but the quality is not always as good.
An example of a top-down process is this one by researchers at Rutgers University. They use sulfuric acid to oxidize graphite flakes. This forces the graphite layers to split apart. They filter out the acid, leaving graphene oxide. Then they remove the oxygen using hydrazine, which leaves pure graphene.
Another method is to grow graphene on a surface, but it is difficult to get the graphene off. The best surface to use is liquid copper because it is easier to remove the graphene. The trouble is, that copper is only liquid at 1000℃.
As more people become interested in the potential benefits of graphene, more and more research money is pouring into it. It won’t be too long before someone finds a way to mass-produce high quality graphene and when that happens, it is going to change our world.
So, graphene is a one-atom thick layer of carbon. It is stronger than steel, harder than diamond, more flexible than rubber, conducts heat and electricity better than copper, and is so light you wouldn’t even know if you were holding some. It will change our lives in the coming years. And this is what I learned today.
Image By AlexanderAlUS – Own work, CC BY-SA 3.0, https://commons.wikimedia.org/w/index.php?curid=11294534
Sources
https://en.wikipedia.org/wiki/Graphene
https://www.nanowerk.com/what_is_graphene.php
https://www.graphene-info.com/graphene-applications
https://www.graphene-info.com/graphene-introduction
https://www.graphenea.com/pages/graphene#.YejP2v7P0dU
https://en.wikipedia.org/wiki/Lamella_(materials)
https://en.wikipedia.org/wiki/P._R._Wallace
https://www.americanscientist.org/article/mass-producing-graphenehttps://www.nanowerk.com/spotlight/spotid=25744.php
