I learned this today. A superfluid is a point where matter behaves like a fluid but has zero viscosity.
A superfluid has no viscosity. That means it doesn’t have any friction and doesn’t lose energy. It appears to go against the laws of thermodynamics. If you stir a cup of coffee with a spoon and step away, the coffee will slowly stop spinning. If you stir a superfluid with a spoon and step away, it will still be spinning a billion years later. Because the superfluid has no viscosity, it loses no kinetic energy.
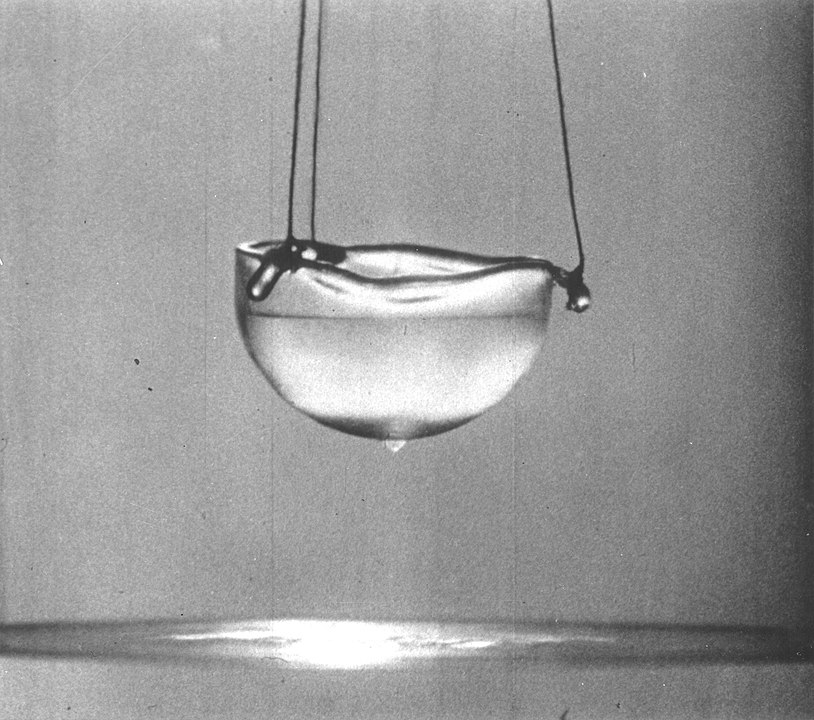
When a liquid is a superfluid, it can flow between molecule thin cracks in the container and it can flow up the sides of the container because of its own internal pressure. It has no friction. If you put a superfluid in a bucket and spin the bucket, the superfluid would not move.
To create a superfluid, a gas has to be cooled to very close to absolute zero. The gas used is helium. Helium becomes a liquid at -269℃. If you can continue cooling it for another few degrees it becomes a superfluid. Absolute zero is -273.15℃. The helium is cooled until it is at -270.92℃. This is done by compressing the gas and then expelling it through a tiny nozzle. The sudden expansion causes the gas to rapidly cool. The cooled gas is fed back into the compressor and the process is repeated until it has cooled enough to become a superfluid.
When the helium gets that cold the atoms undergo a change that gives them this frictionless property. Friction is caused because the irregularities in one object come into contact with the irregularities on the edge of another object. These collisions cause the transfer of energy and the moving object loses its kinetic energy.
In a liquid, the viscosity makes it lose its kinetic energy. When we stir our cup of coffee again, the coffee has a certain viscosity. Viscosity is the measure of a fluid’s resistance to deformation. When fluids move, they do so in layers. They don’t move as a complete object. When we stir the coffee in our cup the coffee layers move at different speeds. The layer in the center moves faster than the layer at the edge. The atoms get in each other’s way and cause resistance. There is a viscous drag force that acts between the fluid layers and the coffee comes to a stop.
A superfluid doesn’t have any viscosity. When helium reaches these incredibly cold temperatures, all of the atoms begin to act as one. Atoms usually vibrate and move a lot. At cold temperatures most atoms lose their energy and become a solid. The atoms are attracted to each other and once their energy has been reduced enough, their attraction overtakes their vibration and they become a solid. Helium doesn’t do this because its atoms are so light and weakly drawn to each other. Even when they are close to absolute zero, they still vibrate a little with something called zero-point motion. Because of this, the individual atoms start to overlap each other until they become one object. They all move at exactly the same time. This means when you spin a bowl full of a superfluid, the atoms don’t collide with each other because they are all moving in unison.
If you drop a hammer into water, the hammer will collide with the water molecules and it will slow down. It won’t lose all of its speed, of course, but it will slow down. If you drop the hammer into an even denser liquid, it will slow down even more. However, if you drop the hammer into a superfluid, it won’t slow down and will hit the bottom at full speed. All of the atoms in the superfluid move out of the way of the hammer at the same time and there is nothing to slow the hammer’s descent.
Helium was first liquified in 1908. People started to notice that helium behaved in strange ways at those temperatures but it was only in 1937 that Pyotr Kapista, a Russian, realized that the helium was a superfluid.
So, when helium is cooled to close to absolute zero, all of the atoms in it overlap each other and it starts to behave as one object. This gives it zero friction, zero friction, and makes it a superfluid. And this is what I learned today.
Photo by Visit Greenland: https://www.pexels.com/photo/aurora-borealis-360912/
Sources:
https://en.wikipedia.org/wiki/Superfluid_helium-4
https://en.wikipedia.org/wiki/Superfluidity
https://news.illinois.edu/view/6367/730694
https://www.sciencefocus.com/science/how-is-helium-turned-into-a-liquid-and-a-superfluid/
https://www.newscientist.com/article/mg20827813-300-first-frictionless-superfluid-molecules-created/
https://www.scientificamerican.com/article/superfluid-can-climb-walls/https://www.aps.org/publications/apsnews/200601/history.cfm
