What is the smallest thing an electron microscope can see? An electron microscope can see atoms that are 0.05 nanometers long. 0.05 nanometers is a very difficult number to imagine. How small is it? Well, a human hair is about 50,000 nanometers across.
There are different types of microscopes. The basic type that students use in schools are called light microscopes. A light microscope uses lenses to bend the light that is passing through the thing you are looking at. The lenses are convex, and they bend the light coming through the sample towards the eye, magnifying it. A powerful light microscope will use several lenses because each one magnifies the light that has gone through the lens before it, greatly increasing the magnification. The most powerful light microscope can see objects that are about 500 nanometers across. The reason for this is that the smallest wavelength of visible light is about 500 nm. If an object is smaller than this, it is too small for the light wave to interact with it.
Electron microscopes can see things that are smaller than this because they use electrons instead of light. To see anything through a microscope, you need to have a wave with a wavelength that is half the size of the object you are trying to see. If the wavelength is too long, it just shoots past the object, in the same way as our light microscope. Because the length of the wavelength gets shorter the more energy they particle has, it is possible to have electrons with a wavelength of less than one nanometer.
The first electron microscope was built in 1931 and it could magnify objects by 400 times. Modern electron microscopes can magnify by up to a million times. Electrons have a variable wavelength that changes by the amount of energy they have. If you can use a high voltage and accelerate the electrons to a very high speed, they can have a wavelength of 1 nanometer, meaning they can hit a 1 nanometer atom and an image of it can be created. The electrons hit the object and they cause the emission of other particles on the other side of the object and the strength and type of these particles depends on what the primary electrons have hit. These particles can be low energy electrons, other high energy electrons, X-rays, and even photons. All of these particles are detected by a screen and analyzed to make an image of the object. The most powerful electron microscopes can even see atoms, but some of the electrons displace the atoms, so it is not always the best way. In 2018, scientists at Cornell University developed a more accurate way of detecting the electrons and were able to see down to 0.39 angstroms (0.039 nanometers) and could clearly see Sulphur atoms. I wonder what possibilities the next advance will bring.
Electron microscopes are difficult to use because they take a lot of setting up. The object being observed has to be in a vacuum because electrons don’t travel very far through the air. The fact that you need a vacuum means that it is impossible to look at live cells. Electron microscopes are also extremely expensive, very large, and require a lot of care.
The future of microscopes will involve making the resolution of the images better. Coupled with that the displays and the software will improve. AI systems will be able to analyze the incoming data more accurately and give a better representation of the object being viewed. AI may also be able to filter out all of the background noise, which will improve the image of the object. There is probably a limit to how far in we can go. If we want to detect things that are smaller than atoms, such as quarks, different methods are needed. The Large Hadron Collider at Cern can detect subatomic particles, but it cannot “see” them. They are detected by the amount of energy that is there, or by the amount of energy that should be there, but isn’t.
So, an electron microscope can see down to about 0.05 nanometers, which is the size of the smallest atom, And this is what I learned today.
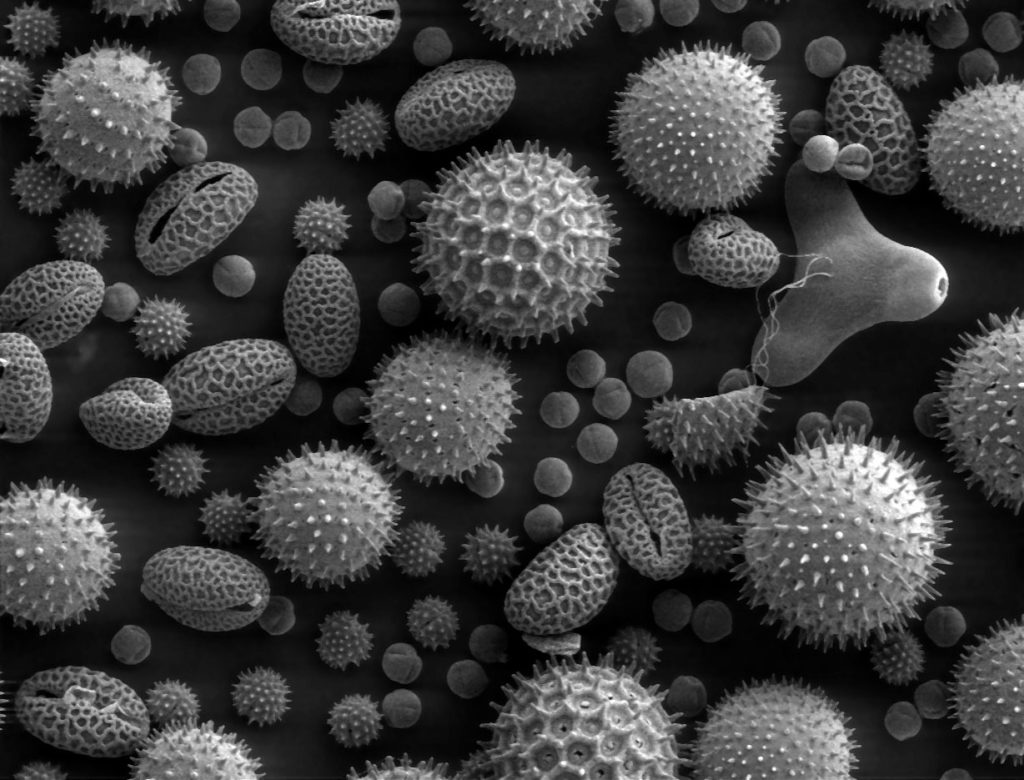
Image By Dartmouth College Electron Microscope Facility – Source and public domain notice at Dartmouth College Electron Microscope Facility ([1], [2]), Public Domain, https://commons.wikimedia.org/w/index.php?curid=24407
Sources
http://scienceline.ucsb.edu/getkey.php?key=7046
https://en.wikipedia.org/wiki/Electron_microscope
https://en.wikipedia.org/wiki/Scanning_electron_microscope
https://www.umassmed.edu/cemf/whatisem/
https://www.sciencelearn.org.nz/resources/497-the-microscopic-scale
https://www.khanacademy.org/science/biology/structure-of-a-cell/introduction-to-cells/a/microscopy
